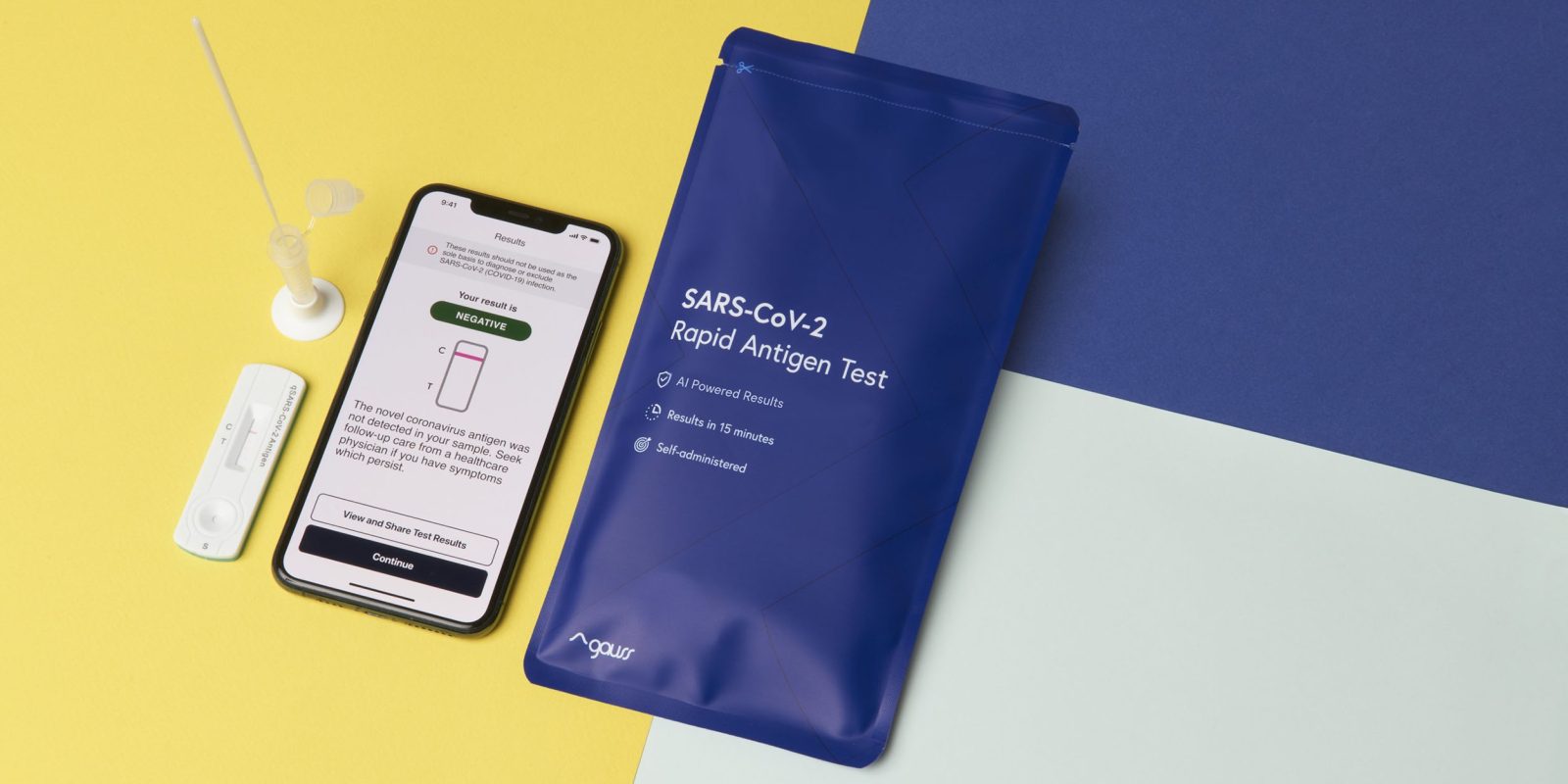
A rapid antigen home COVID-19 test is a purely standalone chemical process, but that hasn’t stopped one enterprising company with a smart marketing team from finding a way to involve an iPhone…
The tests work like this:
- You take a swab from the back of your throat and inside your upper nasal tract
- Place the swap into a vial of liquid
- Drop some of the liquid onto a unit similar to a home pregnancy test
- Wait 15-30 minutes for one or two lines to appear
There are two labels next to the test-strip: C and T. C stands for Control, and the line next to that should always appear (or else the test isn’t valid). T stands for Test, so no line next to that at the end of the waiting period is a negative test, while a line is a positive test.
This is not a difficult test to read – but Kroger Health thinks we need an AI camera app to help.
“Since the start of the COVID-19 pandemic, Kroger Heath has remained committed to helping people live healthier lives by offering a variety of COVID-19 testing solutions supported by our multi-disciplinary team of licensed, trained and experienced healthcare providers,” said Colleen Lindholz, president of Kroger Health. “We’re proud to partner with Gauss to expand our COVIDCare+ suite with the forthcoming launch of this affordable, innovative solution.”
To use the test, patients follow simple step-by-step video instructions in an app to properly collect the nasal swab and complete the rapid antigen test. After 15 minutes, the app prompts the patient to scan their rapid test. The app uses patent-pending, artificial-intelligence-based technology to provide patients with their results in seconds, helping minimize reader variability. To fulfill legal reporting requirements, the consumer-friendly app seamlessly also shares the reliable, secure and HIPAA-compliant results with appropriate public health agencies […]
The test, developed by Gauss and Cellex, Inc., a biotechnology company specializing in rapid diagnostics, is powered by an encrypted, HIPAA-compliant, easy-to-use smartphone app developed by Gauss’s team of clinical and technology leaders. According to results of a clinical trial submitted to the FDA in support of the solutions EUA application, the testing solution demonstrated a 93% positive agreement and 99% negative percent agreement compared to high-sensitivity, emergency-use-authorized PCR tests.
So basically the camera gets an image of the test strip and the app tells you whether or not there’s a line next to the T…
Ten out of 10 for marketing, though!
Kroger says the kit is currently awaiting FDA authorization, and will then be sold online and over-the-counter and online at the company’s 2,200 pharmacies across the US.
FTC: We use income earning auto affiliate links. More.



Comments