FDA begins cracking down on medical diagnosis apps starting with ‘uChek’ iPhone urinalysis app

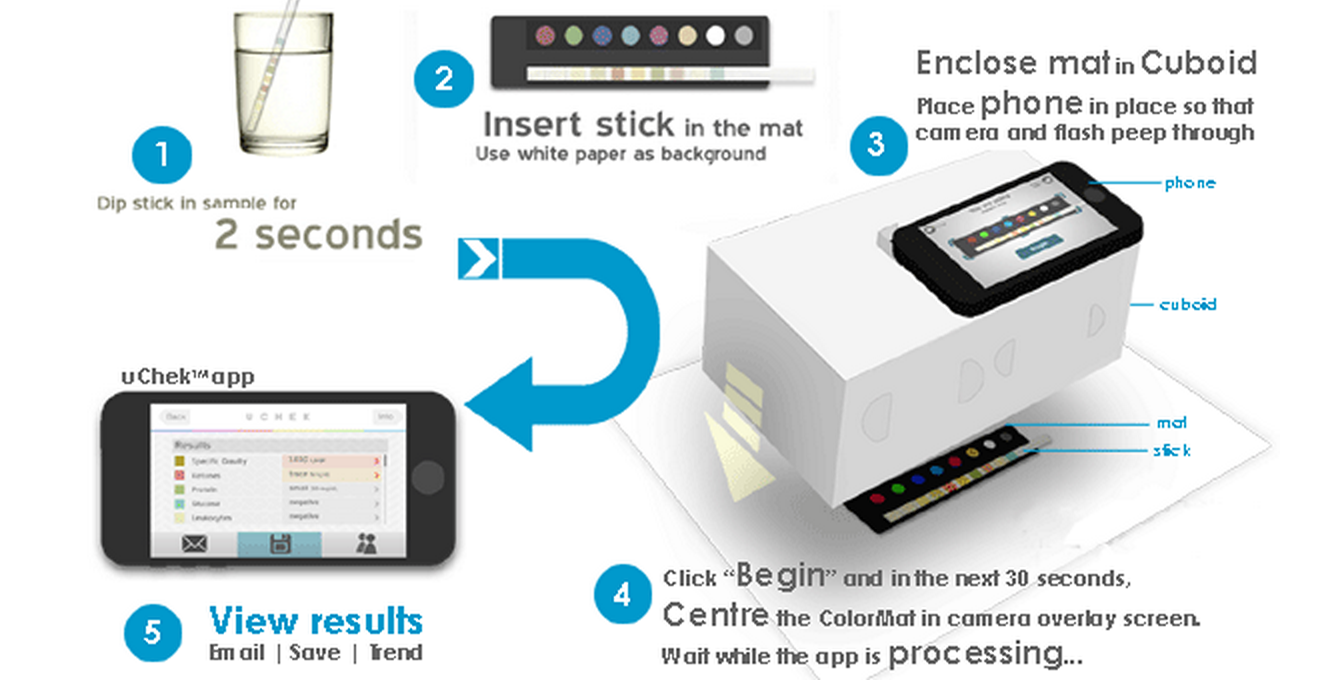
Bloomberg reports the U.S. Food and Drug Administration is launching a first of its kind inquiry into medical diagnosis apps starting with the ‘uChek’ urine analysis app from Biosense Technologies. The free app, which is currently still on the App Store, requires users to purchase a kit containing urine test strips that can be visually analyzed with the iPhone’s camera. The problem, according to a letter sent to Biosense from the FDA, is the fact that the test strips have only been cleared for “direct visual reading” and not automated analysis from an application:
Please note that though the types of urinalysis dipsticks you reference for use with your application are cleared, they are only cleared when interpreted by direct visual reading. Since your app allows a mobile phone to analyze the dipsticks, the phone and device as a whole functions as an automated strip reader. When these dipsticks are read by an automated strip reader, the dipsticks require new clearance as part of the test system. Therefore, any company intending to promote their device for use in analyzing, reading, and/or interpreting these dipsticks need to obtain clearance for the entire urinalysis test system (i.e., the strip reader and the test strips, as used together).
While Biosense plans to work with the FDA to resolve the issue, Bloomberg notes that this is only the start of a broader crack down on apps that claim to diagnose medical conditions:
Expand
Expanding
Close