Scanadu turns your iPhone into an electrocardiogram, urinalysis reader and future drug testing device

[youtube=https://www.youtube.com/watch?v=3cE9Xc5kqmY&HD=1]
Scanadu cofounder Sam De Brouwer demonstrates the Scout and Scanaflo
At CES this week I met with a very interesting company called Scanadu which makes two interesting healthcare products that connect with the iPhone…
 The Scanadu Scout (pictured, right) is a little electronic device designed by Yves Béhar that you touch to your forehead for a few seconds. Almost instantly, physiological parameters, including temperature, heart rate, blood oxygenation, respiratory rate, ECG, and diastolic/systolic blood pressure are sent to an app on your iPhone which logs these measurements and alerts users to anomalies and deviations which may be cause for heath concerns.
The Scanadu Scout (pictured, right) is a little electronic device designed by Yves Béhar that you touch to your forehead for a few seconds. Almost instantly, physiological parameters, including temperature, heart rate, blood oxygenation, respiratory rate, ECG, and diastolic/systolic blood pressure are sent to an app on your iPhone which logs these measurements and alerts users to anomalies and deviations which may be cause for heath concerns.
The Scout closed a $1.6M Indigogo funding round in 2013 and is still trying to push the product through the FDA as it tries to get deliveries to customers.
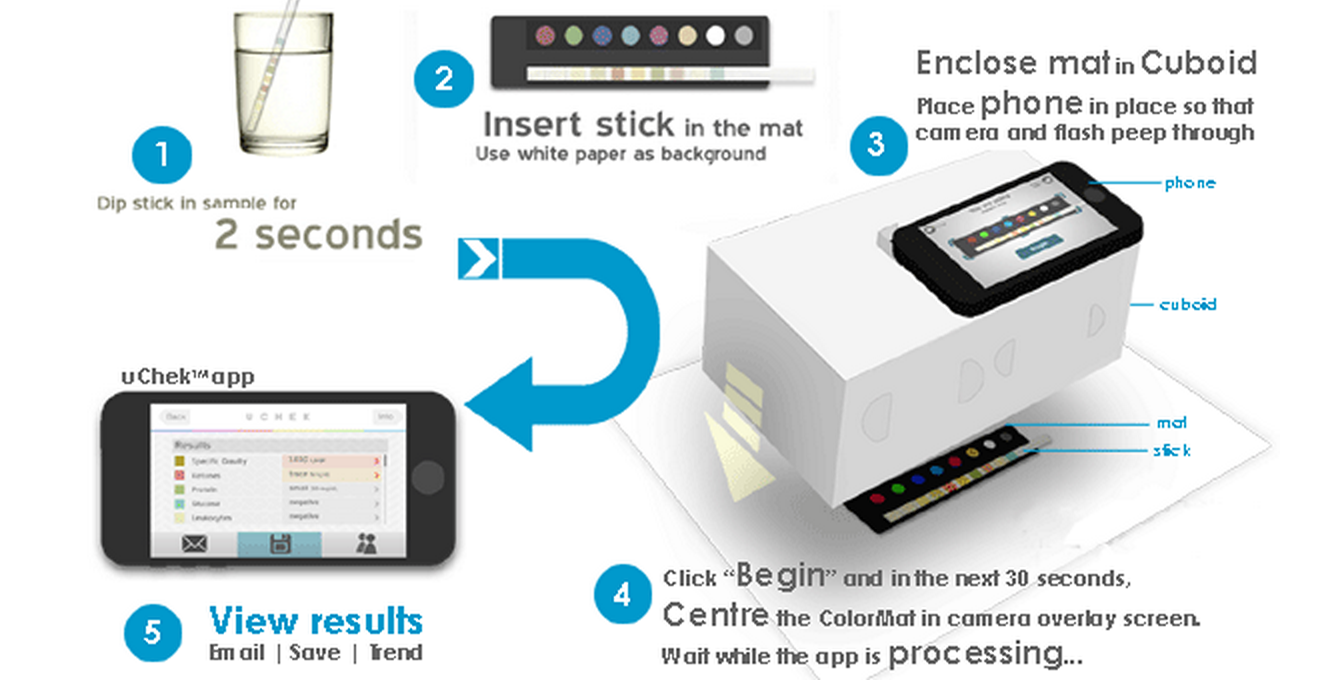
Perhaps more interesting however, Scnadu introduced its new “Scanaflo” device at CES 2015 which is a home urinalysis apparatus that uses your iPhone’s camera to image a set of colors strips.
Expand
Expanding
Close